Abstract
Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic disorders characterized by morphological dysplasia, cytopenias and high risk of progression to acute myeloid leukemia (AML). Despite recent improvements in treatment strategies, the 5-year relative survival rate in MDS remains only 31%, dropping to 24% in AML. This highlights the need for a deeper characterization of the pathogenic mechanisms driving disease development and progression.
Splicing factor (SF) mutations are the most common genetic abnormality in MDS and secondary AML. These mutations, in particular U2AF1 mutations, are associated with increased risk of AML transformation and poor survival. U2AF1 S34F and Q157R mutations determine RNA splicing alterations that eventually result in ineffective hematopoiesis. However, molecular mechanisms and biological consequences underlying the clonal advantage of SF-mutant over wildtype cells remain to be fully elucidated.
To address this aim, we recently combined high-resolution transcriptomics and imaging techniques in preclinical models of U2AF1-mutant and wildtype MDS/AML (Biancon et al., Mol Cell 2022). Our results showed that U2AF1 mutations cause widespread RNA binding and splicing alterations that directly affect the biology of RNA granules, i.e. dynamic condensates of RNAs and RNA binding proteins implicated in RNA metabolism, signaling and gene regulation. In particular, in U2AF1-mutant vs WT cell lines and primary cells we observed increased formation of stress granules (SGs), cytoplasmic RNA granules mediating cellular adaptation to stress conditions. Crucially, the enhanced stress response in U2AF1-mutant cells was associated with improved cell fitness, that was reverted by SG inhibition. Dysregulation of SGs, and potentially of other subcellular RNA condensates, may therefore represent a novel therapeutic vulnerability in MDS/AML patients carrying U2AF1 mutations.
We here integrated single-cell (sc)RNA-seq data to further support the direct link between the U2AF1-mutant genotype and the increased SG phenotype in myeloid malignancies. We first isolated CD34+ blasts from bone marrow samples of MDS patients with (n=3) or without (n=3) U2AF1 S34F mutation. We then performed 10x Genomics scRNA-seq obtaining an average of 3,478 sequenced cells per sample for downstream analyses. Cells from different patients were integrated with Seurat v4.0.1 and annotated with the recently released Cell Marker Accordion (https://github.com/TebaldiLab/TheCellMarkerAccordion), confirming the expected skewing towards granulocyte-monocyte progenitor cells and erythroid lineage in S34F-mutant patients.
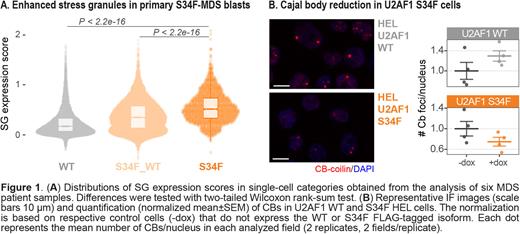
Single cells were subsequently classified according to the detection of the S34F mutation (VarTrix v1.1.19), into: "WT" (WT cells in U2AF1-WT patients), "S34F_WT" (WT cells in U2AF1-S34F patients), "S34F" (S34F-mutant cells in U2AF1-S34F patients). Each cell was also associated to an "SG expression score", measuring the collective abundance of 149 transcripts enriched in SGs and aberrantly bound-spliced by mutant U2AF1 (Biancon et al., Mol Cell 2022). Importantly, S34F-mutant cells represented the category with the highest SG expression score (Figure 1A), consistently indicating an increased stress adaptation that might explain the clonal advantage of splicing factor-mutant cells in MDS.
To further understand the effect of U2AF1-mutant binding and splicing alterations on other RNA condensates, we focused on Cajal bodies (CBs), nuclear granules involved in snRNA transcription and assembly of spliceosomal RNPs. Immunofluorescence (IF) staining for coilin, a known CB marker, followed by confocal imaging revealed a decrease in the number of CBs in U2AF1 S34F vs WT myeloid cell lines (Figure 1B). Of note, IF data were supported by RNA-seq data performed in the same cellular system (Biancon et al., Mol Cell2022), showing a significantly lower expression of snRNAs in U2AF1 mutant vs WT cells. The role of CBs in cancer is currently unknown, but the disruption of CBs may be associated to altered RNA splicing kinetics and increased genomic instability.
Collectively, our results suggest that U2AF1 mutations affect the compartmentalization and aggregation properties of targeted RNAs, providing the rational to further investigate RNA granule perturbations in splicing factor-mutant myeloid malignancies for therapeutic potential.
Disclosures
Halene:Forma Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal